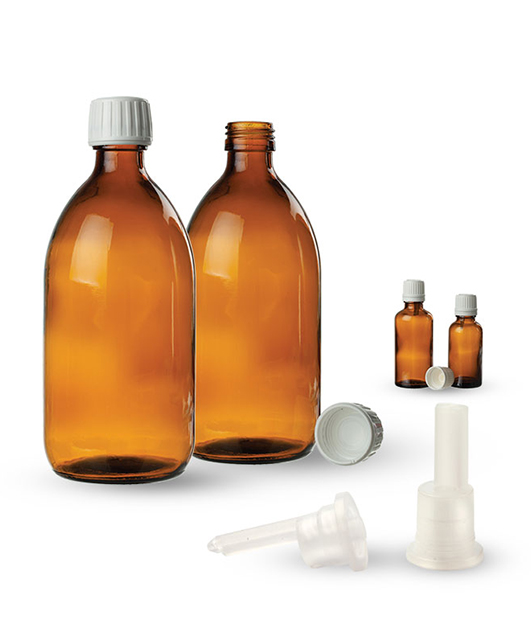
Type III multi-dose glass containers (made from natron-silicate tainted glass which has moderate hydrolytic resistance) are used in pharmacy and they meet the requirements of the assessment defined by pharmacopoeia. They are designed to be in direct contact with the pharmaceutical products. These containers are „tamper-proof“(closures with tamper-evident band) because they ensure that the container is original and not opened. The closures (inner and outer) are made from polyethylene and they contain approved additives. The pharmaceutical plastic containers are made from materials that are described in Ph.Eur. and they meet the criteria of the specific use assessment that are also described in Ph.Eur. We buy the materials from highly reliable producers. The conditions and the method of production of plastic containers and closures are in accordance with the following standards: ISO 9001, ISO 14001, and OHSAS 18001. With the containers we deliver producer´s certificate that is accompanied by technical documentation and documentation which states that the final products are safe for health.